• Mechanical Type- McLeod Gauge
• Thermal Type- Pirani Gauge and Thermocouple
• Ionization Type- Hot Cathode and Cold Cathode
• Radiation vacuum Gauge- Alphatron, Quartz Reference
McLeod Gauge

• McLeod Gauge is a device that is used to measure a pressure at a very low range up to 10-6 torr.
• McLeod Gauge is used to measure vacuum pressure.
• It was invented by British scientist Herbert McLeod in the year 1874.
• It is similar to a mercury manometer. It uses mercury to measure fluid & height of the mercury column determines pressure difference.
Principle
It basically works on the principle of Boyle’s law.
Boyle’s law:- For a gas of a certain quantity at a constant temperature, the pressure of a gas varies inversely with respect to the volume of gas.
- Pal/V (at constant T
- PV = constant
- P1V1=P2V2
Now in this case gas of known volume with unknown pressure is compressed in measuring capillary at a constant temperature.
Construction
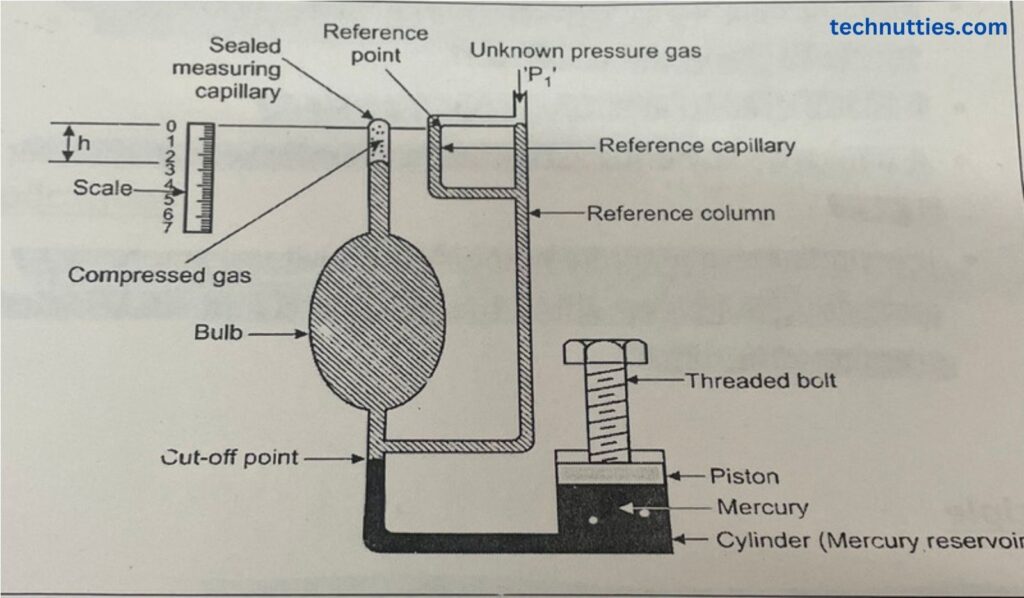
The McLeod gauge consists of the following parts:-
- Working Fluid or Measuring Fluid:- In this measurement, mercury is used as a working fluid for compression of the gas at an unknown pressure. The level of mercury should be below the cut-off point at the start of the procedure.
- Mercury (Hg) Reservoir:- The purpose of the reservoir is to store mercury. It contains a piston or plunger that pushes the mercury into the reference column & bulb & measuring capillary for compression of gas trapped in the bulb & capillary.
- Reference Column:- One end of the reference column is attached at the cut-off point & another end is used as an entrance for gas whose pressure is to be measured. The gas first enters into the Reference column & then enters into the bulb from a cut-off point. The diameter of that Reference column is greater than the diameter of the Reference capillary tube.
- Reference Capillary:- Both ends of the reference capillary are connected to the reference column. It is constructed in such a way that it should be nearest to measuring capillary. The function of the reference capillary is to measure the difference in the levels of both tubes. The diameters of the measuring capillary & reference capillary should be the same & less than the diameter of the reference column to reduce the errors.
- Bulb:- It should be connected to the reference column at the cut-off point. The second end is connected to the measuring capillary. The volume of the bull is considerably higher than the volume of the measuring capillary to take the maximum volume of gas & to increase accuracy while pressure measurement.
- Measurement Capillary:- The top end of the measuring capillary is created is sealed & another end is connected to the bulb. The measuring capillary is constructed closer to the reference capillary & scale is fixed in between the measuring capillary & reference capillary to measure the difference in levels of mercury.
Working
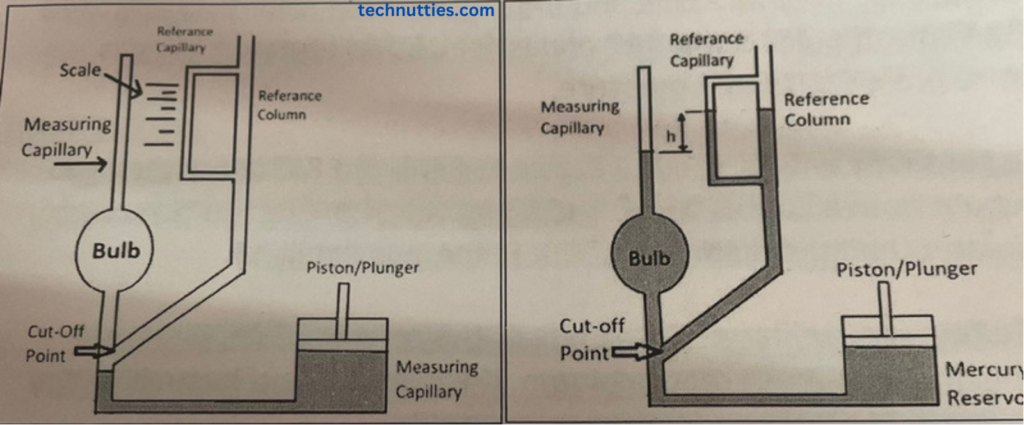
- Firstly, remove the mercury in the bulb & reference the column below the cut-off point so that gas can enter into the bulb & capillary. The piston moves in an upward direction that’s why it causes the suction, so mercury comes into the mercury reservoir.
- Connect the reference column to the source of the gas or vacuum whose pressure is to be measured.
- After filling of gas into the bulb & measuring the capillary, start the filling of mercury into the bulb & reference column by moving the piston downward.
- When mercury crosses the cut-off point, the gas trapped in the bulb & measuring capillary starts compressing.
- Fill the mercury till it reaches zero reference point on the reference capillary.
- After reaching the mercury up to the zero reference point in a capillary, measure the difference in levels between the measuring capillary & reference capillary. It is denoted by ‘H’.
- By knowing this height ‘H’, we can calculate the final volume of gas.
- It also gives the difference between final & initial pressure.
- Now by using Boyle’s law, we can find the unknown pressure of the gas.
Calculations
Now as V1, V2 and P2 are known;
the applied pressure P1 should be calculated using Boyle’s Law given by:
P1V1 = P2V2
Let the volume of the bulb from the point cutoff up to the beginning
of the measuring capillary tube = V
Let area of cross-section of the whole measuring capillary tube = a
Let height of whole measuring capillary tube = hc.
Thats why, initial Volume of gas entrapped in the bulb plus
measuring capillary tube = V1 = V + ahc
When mercury is forced upwards to reach the zero reference point in
the reference capillary, the final volume of the gas = ah = V2.
where,
h = height of the gas which is compressed in the measuring capillary tube
P1 = Applied pressure of the gas that is unknown.
P2 = Pressure of gas at last condition, that is,
after compression – P1+h
From Boyle’s Law
P1V1 = P2V2
P1V1 = (P1+h)(ah)
P1 = Ah2/(V1-ah)
P1 = Ah2/V1 (neglecting ah, because V1>> an)
Therefore, the applied pressure is calculated using McLeod pressure.
Advantages
- It is independent to the composition of the gas whose pressure is to be measured.
- McLeod Gauge is used to reference standards to calibrate other pressure gauges as the value of that pressure measured by this gauge is very accurate.
- In this, there is no need to correct the readings of this gauge.
- A linear relationship exists between applied pressure and ‘h’.
Disadvantages
- The gas for which pressures are to be measured must obey
Boyle’s Law. - A moisture trap is a must to prevent any vapor from entering the gauge; otherwise, the vapor will cause an error in the reading of the gauge.
- It measures only on a sampling basis.
- McLeod Gauge cannot give a continuous output.
- There is a chance that it can be get contaminated easily.
Applications
- McLeod gauges are basically used for the calibration of other inferior types of gauges.
- The vapor pressure of Mercury determines only the lower limit of the measurement range of that gauge.
